

Currently the standard is the nucleus of a carbon atom containing six protons and six neutrons (carbon-12). Gold, fluorine, aluminum and sodium, each of which exists in only one stable version, will be left alone.Ĭredit (from left to right): Scewing/Wikimedia Commons Kuebi/Wikimedia Commons Mav/Wikimedia CommonsĪtomic weight refers to the averaged mass of the atoms of a chemical element using a scale based on a standard atomic nucleus. Now that it has completed the initial round, the International Union of Pure and Applied Chemistry’s commission in charge of atomic weights will reassess the rest of the elements in the coming years. “It should have been done a decade ago,” Coplen says. These numerical tweaks implied that the numbers couldn’t be pinned down with precision, when in fact such measurements are more precise than ever, says Coplen, who headed the international task force charged with surveying various isotope abundances in nature so that the numbers could be revised. But as the number of discovered isotopes grew - there are more than 2,000, but only 118 elements - weights kept needing adjustment. Previously, a given element’s official atomic weight was actually an average of this variation. “Isotope studies extend from studies of previous climates to dating artifacts to weapons programs and biomedical applications,” says James Adelstein, a professor at Harvard Medical School and coeditor of a National Research Council report on isotopes in medicine and the life sciences. Similarly, testosterone supplements are plant-derived and have a different isotopic carbon signature than testosterone made by the body (to Tour de France cyclist Floyd Landis’ chagrin). This variation is what makes isotopes such a powerful scientific tool: the relative ratios of the different carbon isotopes can tell scientists if ivory came from an elephant that ate shrubby savanna plants or woody jungle trees. Geological Survey’s Reston Stable Isotope Laboratory in Virginia.įor example, ratios of the three oxygen isotopes will differ depending on whether the oxygen is in air, groundwater, fruit juice or bone. For example, it’s commonly said that more than 99 percent of oxygen is the normal eight-neutron isotope - called oxygen-16 - while the heavier versions exist in fractions of one percent.īut those proportions aren’t set in stone, and the new adjustment to the official weights acknowledges that, says Tyler Coplen, head of the U.S. These heavier versions, or isotopes, have been presented as existing in constant quantities no matter the source. But oxygen can gain an extra neutron or two, changing the element’s weight (electrons are also variable but so light that their weight isn’t taken into account). For example, oxygen, the most abundant element in the Earth’s crust, is most comfortable having eight neutrons and eight protons in its nucleus (the latter of which defines it as oxygen). Most elements have a preferred, energetically stable form that dominates in nature. The change, long overdue, explicitly acknowledges the fact that most of the 118 elements come in multiple forms of varying weight.
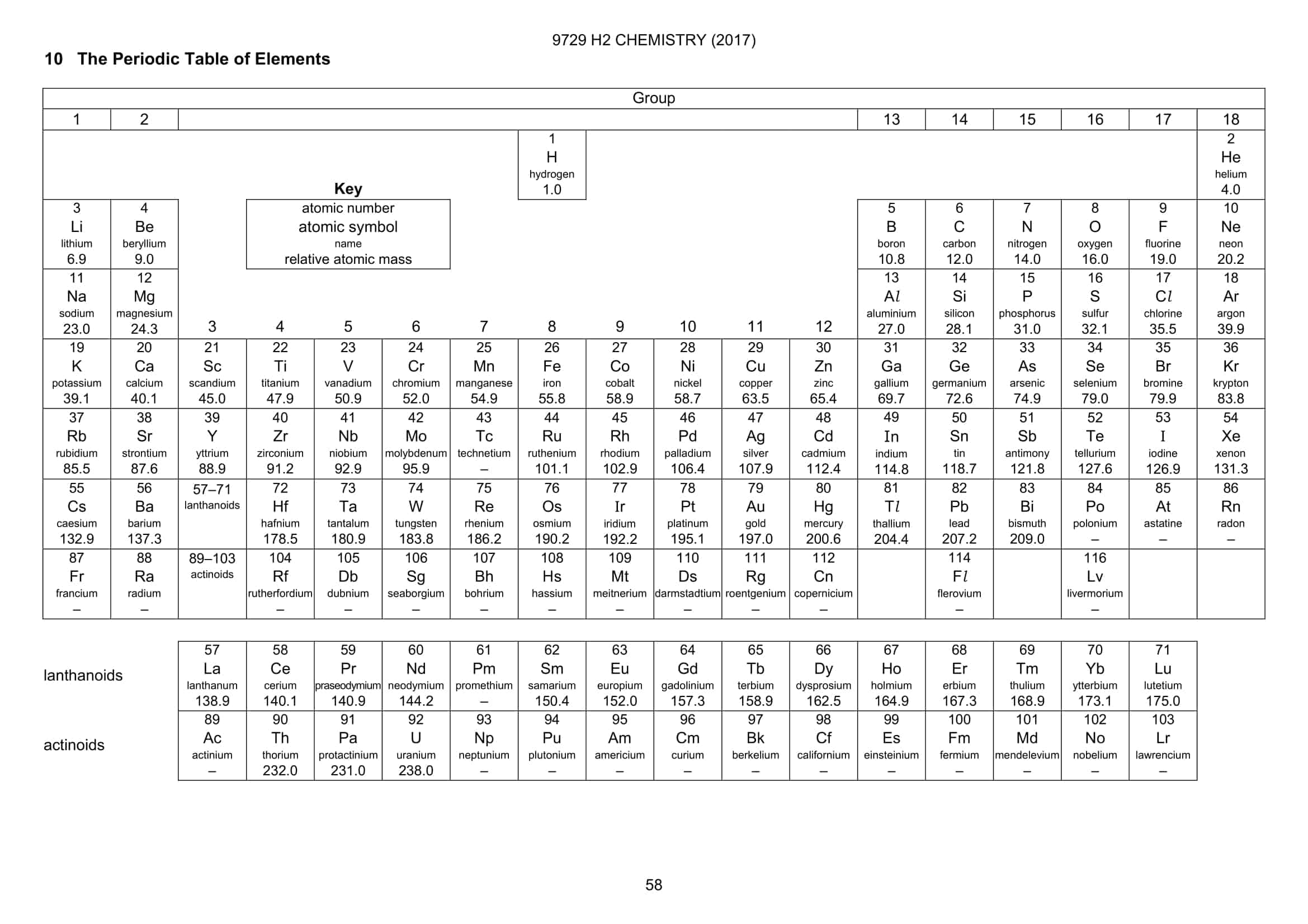

Instead of being described by a single fuzzy number, the atomic weights of oxygen, hydrogen, lithium, boron, carbon, nitrogen, silicon, sulfur, chlorine and thallium will now be expressed as intervals. RESETTING THE TABLE In a new version of the periodic table, the atomic weights of elements with more than one stable form, such as chlorine, are shown as a range, while elements with one stable form, like arsenic, have an exact weight. The adjustments, published online December 12 in Pure and Applied Chemistry, are the first in an overhaul of the atomic weight of almost every element on the periodic table. In acknowledgment of this natural variation, the official weights of 10 chemical elements will no longer be expressed as single numbers, but as ranges.
#Free carbon periodic table square license
Just as the weight listed on your driver’s license doesn’t necessarily reflect your actual poundage, the official atomic weights of most chemical elements are actually more like ballpark estimates than precise constants.


 0 kommentar(er)
0 kommentar(er)
